Patients
Patients
“THE LIGHT OF THE EYES REJOICES THE HEART”
(proverbs 15:30)
At Tarsier, we are motivated to cure blinding diseases of the eye, even those that are rare. We are committed to help patients save their eyesight and see their future.
For this goal, we are working to develop safe and effective drugs for a broad range of inflammatory ocular diseases, with the first target disease being non-infectious uveitis.
We would love to have you involved. Join our community by following us on Twitter and Linkedin for updates, or leave us a note at [email protected].
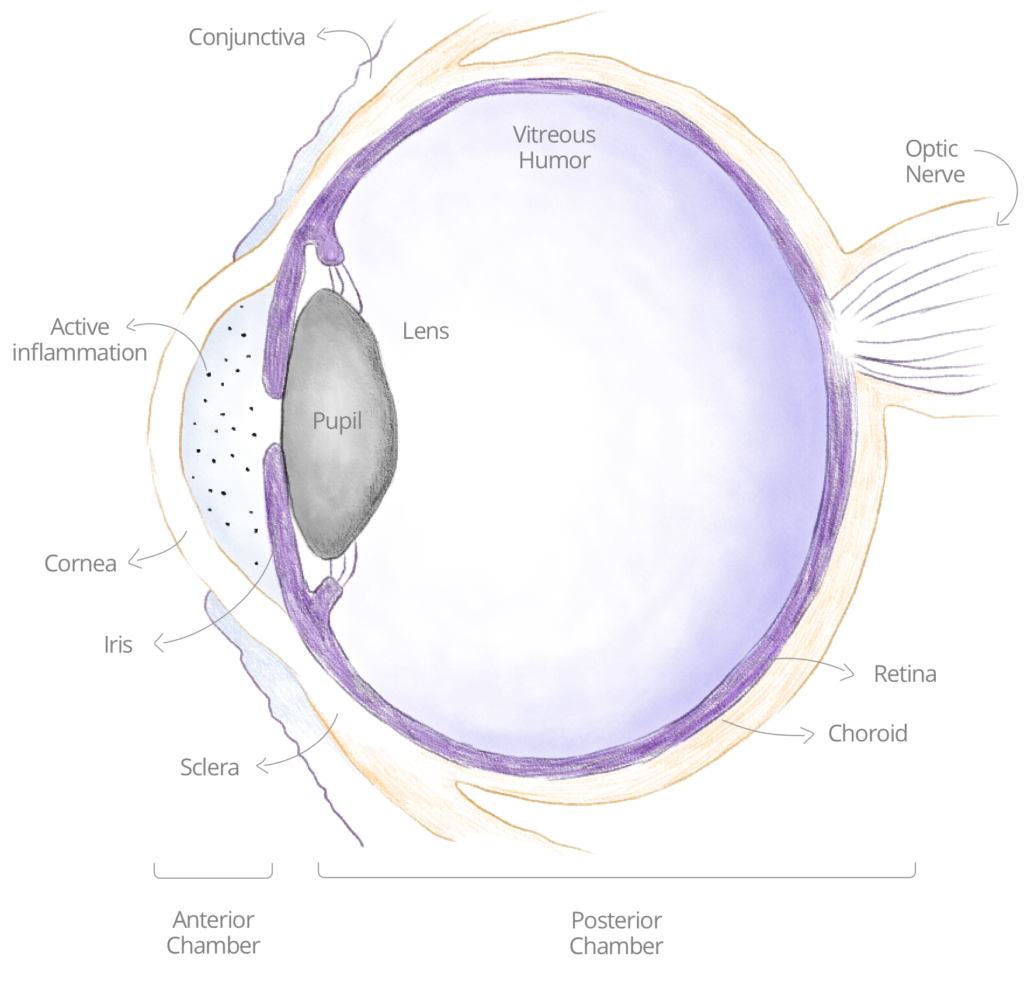
UVEITIC INFLAMMATION
Inflammation in general, and in the eye specifically, is a biologic response of the immune system to harmful stimuli, with the goal of initiating a healing process. This response is triggered through a series of successive chemical signals, called inflammatory cascades, which comprise various types of immune cells. In noninfectious autoimmune uveitis, an inflammation activation in the eye arises even without a genuine harmful stimulus, when the body ‘mistakenly’ attacks self-cells. Such a uveitic ocular inflammation would begin with different types of immune cells (white blood cells), and through a cascade of events, deteriorate to a uveitic flare-up.
During a flare-up, patients may experience pain, light sensitivity/photophobia, eye redness, decreased vision, blurred vision, or “floaters”. If not treated properly and promptly, uveitic flare-ups may lead to blindness.
NON INFECTIOUS ANTERIOR UVEITIC GLAUCOMA
For our first clinical indication, we have chosen to focus on uveitis glaucoma (see also on Wikipedia), for patients who currently have no sufficient treatment alternatives for maintaining their long-term eyesight. Uveitis glaucoma occurs when a patient develops glaucoma on top of uveitis, and it complicates the management of the disease. 30% of patients with uveitis will also develop glaucoma.
Treatment of uveitis addresses aspects of inflammation, pain and structural adhesions formed between adjacent structures within the eye. Long-term management constitutes preventive measures to avoid relapses. Once the disease progresses onto uveitic glaucoma, the first line treatment option and the only approved topical treatment – corticosteroids – should be avoided due to its side effect of increased ocular hypertension.
Our first eye-drop drug candidate, TRS01, is intended to serve as a potent and safe anti-inflammatory agent. Up to now we have completed two Phase I\II clinical trials in the US testing TRS01, with a few dozens of patients. Currently we are recruiting patients for our phase-3 trial, TRS4VISION, in patients with active non infectious anterior uveitis including patients with uveitic glaucoma. All our trials, past and current, can be found on ClinicalTrials.gov of the NIH.
At this time, Tarsier does not have an Expanded Access Policy. As the development of our programs evolve, we will periodically reassess our policy and post any updates to our website. For more questions, please contact [email protected].
POSTERIOR, PAN AND INTERMEDIATE UVEITIS
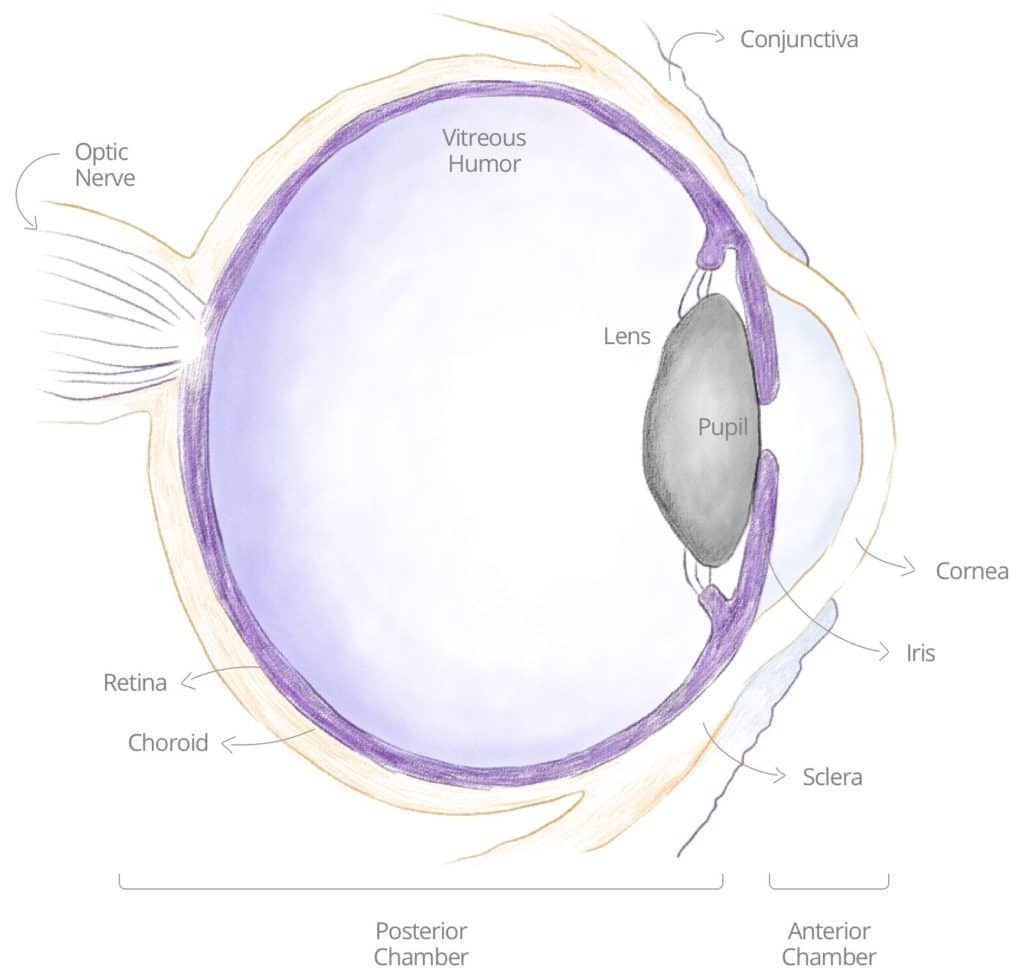
The different types of uveitis are named according to their different primary sites of inflammation. Posterior uveitis means that the primary site of inflammation is in the back of the eye, primarily with the retina or the choroid. The retina is the tissue at the back of the eye which is light-sensitive. The choroid is primarily a vascular structure supplying the outer retina. In intermediate uveitis, the center of inflammation is in the vitreous, which is the gel structure that occupies 80% of the ocular volume. Pan uveitis is when all three major components (anterior, posterior, intermediate) are affected by inflammation.
Posterior, Pan and Intermediate uveitis are also considered rare and serious sight-threatening ocular diseases, with similar unsatisfactory treatments.
In order to optimally reach the back of the eye, our second drug candidate is an intravitreal injection, TRS02, a slow-release formulation of the active pharmaceutical ingredient Dazdotuftide (TRS), and hence has the potential to serve as a potent anti-inflammatory agent without the negative side effects. This product is currently in pre-clinical stages of development.