Meet us at ARVO 2020 in Baltimore
Tarsius Pharma is attending the Association for Research in Vision & Ophthalmology 2020 Annual Meeting that will be held in Baltimore, MD, USA.
Contact us if you wish to arrange a meeting.
Meet us at ARVO 2020 in Baltimore
Tarsius Pharma is attending the Association for Research in Vision & Ophthalmology 2020 Annual Meeting that will be held in Baltimore, MD, USA.
Contact us if you wish to arrange a meeting.



Tarsius Pharma has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 879598
Tarsier® Pharma has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 879598

© Tarsier® Pharma Ltd 2023
A Phase 3 clinical trial of a steroid-free eye drop (TRS01) in the treatment of subjects with active non-infectious anterior uveitis including subjects with uveitic glaucoma. Throughout the duration of this randomized, double-masked study, the safety and efficacy of TRS01 eye drops will be evaluated and compared to standard of care steroid eye drops. Participation is limited to subjects 70 years of age or younger (including children of all ages) who have active non-infectious anterior uveitis and are currently without treatment or on a stable dose of their current treatment. Subjects will be followed for 6 weeks over the course of 6 office visits.
Would you like to participate? You are welcome to answer TRS4VISION ELIGIBILITY QUETIONNAIRE or submit any inquiry to [email protected]
To learn more about the trial and to find out which clinical sites are on board, in the US and in Europe, please visit: Clinicaltrials.gov or visit the US Sites map.
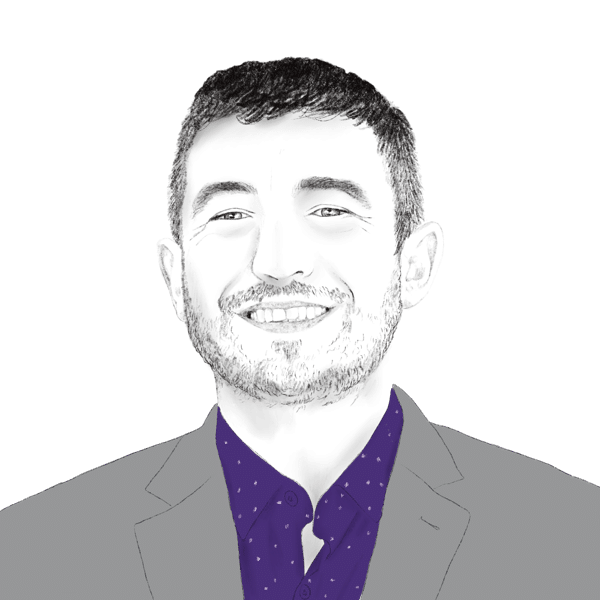
Mr. Haykin brings expertise in peptide drug development with a proven track record in the chemical development of peptide-based APIs, from small R&D scales up to large-scale commercial manufacturing.
Prior to joining Tarsier Pharma, Mr. Haykin served in several positions at Novetide (partially owned by Teva Pharmaceuticals), a leading worldwide peptide manufacturer for the pharmaceutical industry. He was leading R&D teams and projects towards successful development of multi-kg scale GMP production of different peptide-based pharmaceutical products.
Mr. Haykin holds an M.Sc. in Biotechnology engineering from the Technion, the Israeli Institute of Technology.

Hatice Nida Sen, M.D., MHS is a Professor of Ophthalmology at the George Washington University, and Senior Director/Clinical Leader at Janssen Retina R&D, Johnson and Johnson. She is also a special volunteer at The National Eye Institute (NEI), National Institutes of Health (NIH). Dr. Sen earned her MD degree at Hacettepe University of Turkey, she then completed her ophthalmology residency at George Washington University, Washington DC; her clinical fellowship at the National Eye Institute, NIH in Bethesda, MD and her Masters in Health Sciences (MHS) in Clinical Research at Duke University, Durham NC.
Dr Sen has been an investigator at the NEI, NIH for 13 years where she was a Lasker Clinical Research Scholar and NIH Distinguished Scholar and the Head of Clinical and Translational Ocular Immunology Section. She was also the Director of the Uveitis Clinic and the Uveitis and Ocular Immunology Fellowship Program at the National Eye Institute (NEI), National Institutes of Health (NIH). As a prominent investigator in the field of uveitis and ocular immunology Dr. Sen led many clinical trials (phase I to IV), natural history studies, developed clinical and laboratory biomarkers and endpoints. She has mentored many clinical fellows, residents, and research students at NIH and GWU. She continues her part-time clinical practice and teaching at George Washington University and NIH.
She is a board-certified ophthalmologist and is a participating member of American Academy of Ophthalmology (AAO), Association for Research in Vision and Ophthalmology (ARVO). She is the immediate past president of American Uveitis Society (AUS) and is in the leadership committees of professional societies such as International Uveitis Study Group (IUSG), American Ophthalmological Society (AOS). She has authored over 180 peer-reviewed articles and many book chapters in highly prestigious textbooks of ophthalmology and most recently has been the editor of a major uveitis text book, Uveitis Fundamentals and Clinical Practice, 5th Edition. She has received many awards for her academic and research activities including Senior achievement and Secretariat awards of the American Academy of Ophthalmology, ARVO Silver Fellows Class of 2022, Prevention of Blindness (POB) Society of Metropolitan Washington Research Award, NIH Bench to Bedside Award, and NIH Lasker Clinical Research Scholar Award.

Emmett Cunningham is an Operating Partner in the Blackstone Life Sciences group. Having joined Blackstone as part of its acquisition of Clarus in December 2018, where he was a Managing Director. Dr. Cunningham joined Clarus in 2006.
Dr. Cunningham has led investments in medical technology and biotechnology, including partnerships with pharmaceutical companies. Prior to joining Clarus, Dr. Cunningham was the Senior Vice President, Medical Strategy at Eyetech Pharmaceuticals, Inc. where he led the team that developed Macugen, a first-in-class product for the treatment of age-related macular degeneration. Prior to Eyetech, Dr. Cunningham was at Pfizer, Inc. (NYSE: PFE). Dr. Cunningham is an internationally recognized specialist in infectious and inflammatory eye disease with over 400 publications.
Dr. Cunningham is a member of the Board of Directors of Galera Therapeutics and the SFJ Pharmaceuticals Group. Dr. Cunningham was the founder and chairman of the Ophthalmology Innovation Summit (OIS) symposium held in conjunction with the annual meetings of the American Academy of Ophthalmology and the American Society of Cataract and Refractive Surgery.
Dr. Cunningham received an MD and MPH in epidemiology and statistics from Johns Hopkins University and a PhD in neuroscience from the University of California at San Diego for work done at The Salk Institute.

Jennifer Thorne, M.D., Ph.D., is the Cross Family Professor of Ophthalmology at the Wilmer Eye Institute, where she is also chief of the Division of Ocular Immunology and Uveitis. Prof. Thorne holds a joint appointment as professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health.
An internationally recognized and board-certified ophthalmologist, Prof. Thorne is an expert in the evaluation and management of patients with uveitis and other related immune-mediated disorders. She has published over 200 articles and book chapters on uveitis and ocular immunology, won approximately 30 scientific and clinical awards, and has participated in numerous uveitis clinical trials.
Prof. Thorne’s research interests include white dot syndromes including birdshot chorioretinitis, multifocal choroiditis and punctate inner choroiditis. She also studies juvenile idiopathic arthritis-related uveitis and treatment outcomes of immunosuppressive drug therapy.
Dr. Thorne is active in American and international Ophthalmology and uveitis-related forums, for example, she was President of the American Uveitis Society and remains part of its leadership team, was a member of the Executive and Steering Committees of the Standardization of Uveitis Nomenclature (SUN) Working Group and played advisory and editorial roles in the American Academy of Ophthalmology, Ocular Immunology and Inflammation journal, and more. She has also served as an advisor and consultant for ophthalmic pharma companies, such as Allergan, AbbVie, Gilead, and more.
Prof. Thorne received her M.D. degree from the University of Virginia and completed her ophthalmology residency at the University of Pennsylvania’s Scheie Eye Institute. She completed her uveitis fellowship at Wilmer and completed her Ph.D. in epidemiology at the Johns Hopkins Bloomberg School of Public Health.
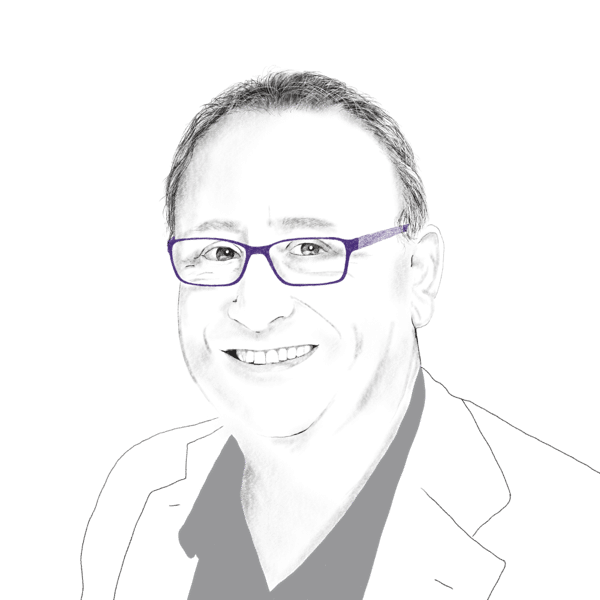
Arie Ganot is an experienced professional in senior financial and executive roles, in private and public companies. Arie has strong capabilities in M&A, IPO, SEC accounting, budgeting, and equity raising. He has led the Mind CTI (Nasdaq: MNDO) successful IPO, a leading provider of billing and customer care solutions, and S.AL Technical Equipment’s IPO at TASE (Tel Aviv Stock Exchange), before its acquisition for 280 million NIS. Formerly, Arie has also been the CFO of Extricom, and a Senior Auditor in PWC. He has founded NatiG CPA, a CFO Services company, in which he serves as external CFO at selected companies. Arie Joined Tarsier’s team as CFO since its establishment in 2016. Arie holds a BA in Economics and Accounting from Tel Aviv University and is a Certified Public Accountant in Israel.

Jonathan Burgin brings to his role as CFO in Tarsier Pharma over 20 years of managerial experience and leadership in public biotech and hi-tech companies.
Most recently he served in the position of CFO & COO at Anchiano Therapeutics, a clinical stage biopharmaceutical company developing drugs against cancer (Nasdaq: ANCN, TASE: ANCN), where he played an instrumental role in building the organization and advancing the lead compound to pivotal clinical trials. He also played an integral role in the Nasdaq IPO and multiple private and public offerings while traded on the TASE raising a total of $96M.
Prior to Anchiano, Jonathan was CFO of Radcom Ltd. (Nasdaq: RDCM), a service assurance provider where he led the global finance and related operations. Prior to that, he was CFO of XTL Biopharmaceuticals Ltd. (Nasdaq: XTLB, LSE: XTL, TASE: XTL), a drug development company, where he played a leading role in four capital transactions raising over $110M. Prior to that, Jonathan was CFO of YLR Capital Markets Ltd., a publicly-traded Israeli investment bank. Earlier in his career, he served as a Senior Manager at PwC Tel Aviv.
Jonathan holds an MBA in finance and a BA in accounting and economics from Tel Aviv University and is a Certified Public Accountant in Israel. He currently serves on the Board of Directors of Cellect Biotechnology Ltd. (Nasdaq: APOP).

Dr. Levin has 25 years of experience developing new chemical entities for US and EU markets, from proof of concept and pre-clinical and clinical stages, through technical transfer, scale-up, and until reaching a commercial phase. She has CMC (Chemistry, Manufacturing and Controls) expertise that includes formulation development (liposomes, sterile injection, semi-solid dosage forms, etc), technical transfer, scale-up and validation in start-up and multinational companies as well as a wide experience in regulatory submissions (IND, NDA, IMPD) for US and EU markets.
Dr. Levin’s experience includes project management, budget preparation, contract manufacturer selection and management, and project risk assessment. She has served as Director of CMC and CMC Lead in Neuroderm, a pharmaceutical company developing treatments for central nervous system (CNS) disorders, and as CMC Project Manager in Dexcel Pharma, which develops, manufactures and markets pharmaceutical products. Dr. Levin has a B.Sc in Chemistry, M.Sc and Ph.D in Biochemistry, and an MBA, all from Tel-Aviv University.

Dr. Eidelman has over 40 years of experience in the field of organic synthesis and peptide chemistry, including chemical and analytical R&D, QC, process development and project management. He has over 25 years of experience in development and production of synthetic peptide APIs (Active Pharmaceutical Ingredients). Dr. Eidelman has served as Chief Chemist and VP R&D in Novetide, today a global company developing peptide synthesis technologies and production processes for the APIs’ pharmaceutical industry. He has been instrumental in the company’s evolvement and growth, from its inception as a new spin-off until its success in large global scales, by building the R&D capabilities to serve manufacturing facilities for peptide products, managing various processes such as tech transfer to manufacturing sites, and scaling-up the activity and team. Beforehand Dr. Eidelman has been in ICL (Israel Chemicals Group), first as a Researcher and Project Manager at Monomers and Fine Chemicals Groups, and then as a Project Manager at the Peptide Group. Between 2015-2020 he has also served as a member of United States Pharmacopeia BIO1 Expert Committee, an international expert committee that designs policy and sets standards of peptide products for the US market. Dr. Eidelman has a B.Sc and M.Sc in Chemistry from the Technion Israel Institute of Technology, and a Ph.D in Chemistry from Tel-Aviv university.

Ms. Friedel brings experience in developing international partnerships for technology and innovation, inter alia as Director of SMEs Department in ISERD (Israeli-European R&D Directorate) and as Global Business Development and International Collaboration Strategist of Israel Innovation Authority. Participated in designing governmental incentives and in management of foreign relations to support Israeli companies’ R&D cooperation with foreign entities. Also served as a Trade Officer at the Industry and Trade Department in the Mission of Israel to the EU in Brussels, Belgium, where she helped promote Israeli commercial activity in the EU. Ms. Friedel has a BA in French and English Language and Literature from Tel-Aviv University, an MA in European Studies from the Hebrew University of Jerusalem, and an MA in Law from Bar-Ilan University.
We at Tarsier Pharma Ltd. (“Tarsier Pharma”, “we”, “us” or “our”) respect your privacy. This Privacy Policy (“Policy”) explains the accepted privacy practices for our online services at our website (“https://tarsierpharma.com/”). It also describes the rights and options available to you with respect to your personal information.
The Website provides content and information about Tarsier Pharma and its research and development of a novel platform for treatment of ocular inflammatory diseases.
You do not have a legal duty to provide the information and providing it is subject to your unconditional consent. Proving the service of online inquiries on our Website will not be available to those choosing not to provide the information requested.
We collect two types of information: information you actively provide us when contacting us or statistical information about use of the Website.
Inquiries. If you wish to send us a query of any kind via our online contact form, you will be required to provide us with your name and email address (“Inquiry Information”). In order to respond to certain queries, we may ask you to provide additional information (such as your telephone number).
Statistical information. When you visit our Website, we may record and collect certain information in relation to your visit and use of the Website, including: IP address (and location), time and date of access, type of browser used, links clicked and the web pages you accessed (“Analytical Information”).
Cookies. We collect Analytical Information through “Cookies”, which are text files, comprised of small amount of data, that are saved on your computer or other device (e.g. smartphone, tablet, etc.) when you use internet and visit various websites. We use cookies for several purposes:
Necessary. Cookies that are strictly necessary for the functioning of the Website. The Website cannot operate properly without these cookies. You can set your browser to block or alert you about these cookies, but some parts of the Website may not function properly.
Statistics. Cookies that help us understand how you and other users interact with our Website by collecting data that does not directly identify you.
The overall personal data outlined above will be referred to as the “Information”.
We use your Information in order to contact you about Inquiries you made and/or to operate the Website.
We may use your Information outlined above for the following purposes:
Operating the Website and providing its features and functionalities;
Contact you about inquiries you made;
Send you email communications concerning our products, programs, developments and other related information. We will send you email communication subject to your explicit consent;
You may ‘opt-out’ of using your data for promotional communications at any time by following the “unsubscribe” link located at the bottom of each message. By doing so, Tarsier Pharma will only delete the Information which is required to contact you for promotional communications, while the rest of the Information you submitted to us which is necessary in order to provide you with the Website’s services will continue to be processed.
Improve and enhance the services and content offered on the Website.
We share your Information mainly with our contractors and service providers, strictly for the purpose of helping us with the internal operation of the Website.
We will not share your Information with third parties, except in the events listed below or when you provide your explicit and informed consent:
We will share your Information with our service providers (such as our hosting providers) who assist us with the internal operations of our Website and our services.
These companies are authorized to use your Information only as necessary to provide these services to us and not for their own promotional purposes. We do not sell your Information to third parties
If you have breached any agreement you have with us (including this Policy), abused your rights to use the Websites, or violated any applicable law, your Information may be shared with the competent authorities, law enforcement and third parties (such as legal counsels and advisors), for the purpose of handling of the violation or breach;
If we are required to disclose your Information by a judicial, governmental or regulatory authority;
We will share your Information with members of our family group of companies, who help us process the data for the purpose set out above;
If the operation of the Websites is organized within a different framework, or through another legal structure or entity (e.g. due to a merger or acquisition), we will share your Information to enable the structural change.
We will store your data for as long as we deem necessary for the purposes detailed in this Policy
We retain personal data for as long as necessary to fulfil the purposes we collected it for (as detailed above), including for the purposes of satisfying any legal, accounting, or reporting requirements. We, at Tarsier Pharma, always consider the type of information collected, the amount of information, how sensitive it might be. Accordingly, we determine the appropriate retention period.
We may also anonymize your Information so that it can no longer be associated with you, in which case we will use such Information without further notice to you.
We implement measures to reduce the risks of damage and unauthorized access or use of information, including when data is transferred outside the E.U.
Security Measures. We implement measures to reduce the risks of damage, loss of Information and unauthorized access or use of Information. We also request our affiliates to implement such measures in order to secure the Information we provide them. However, although efforts are made to secure your Information, we cannot guarantee its absolute protection.
Cross-Border Data Transfers. We are based in Israel. Information we collect from you will be processed in Israel, which is recognized by the European Commission as having adequate protection for personal data. Certain information may be stored externally via cloud services. We will only transfer your personal information to a country or company which has been deemed to have an adequate level of data protection by the EU commission, or under other adequate safeguards determined under the applicable law.
Controller. Tarsier Pharma Ltd. is the data controller for the purposes of the personal data we collect via the Website and for the performance of the services offered through the Website.
The legal basis for processing and collecting Inquiry Information is its necessity for the provision of feedback, comment or service in response to your inquiry;
The legal basis for processing and collecting Analytical Information is our legitimate interests in operating our website, ongoing management of our business and business development;
The legal basis for collecting and processing your information for Promotional Communications is your explicit consent;
Right to Access. You have a right to access your personal data that we process and receive a copy of it.
Right to Rectification. You have the right to ask us to rectify inaccurate personal data concerning you and to have incomplete personal data completed.
Right to Data Portability. You have a right to receive the personal data that you provided to us, in a structured, commonly used and machine-readable format. You have the right to transmit this data to a third party. Where technically feasible, you have the right that your personal data be transmitted directly from us to a third party you designated.
Right to Withdraw Consent. You have the right to withdraw your consent for processing your personal data at any time. If you do that, we will not collect any further personal data, but we will further process the data we already collected for reasons described in this Policy. Withdrawing your consent will not affect the lawfulness of data processing we carried out based on your consent before such withdrawal.
Right to Object. If you previously agreed that your personal data may be used for other purposes other than registering and/or placing an order, you may have a right to object to the use of your personal data for such additional purposes.
Right to Restrict. You have the right to restrict processing of your personal data (except for storing it) if you contest the accuracy of your personal data, for a period enabling us to verify its accuracy; if you believe that the processing is unlawful and you opposes the erasure of the personal data and requests instead to restrict its use; if we no longer need the personal data for the purposes outlined in this Policy, but they are required by you to establish, exercise or defence relating to legal claims, or if you object to processing, pending the verification whether our legitimate grounds for processing override yours.
Right to be Forgotten. Under certain circumstances, such as when you withdraw your consent, you have the right to ask us to erase your personal data. However, we may still process your personal data if it is necessary to comply with a legal obligation we are subject to under laws in EU Member States.
If you believe your right have been infringed, you can lodge a complaint with a supervisory authority operating under the GDPR. For a list of supervisory authorities in the EU, click here.
Representation for data subjects in the EU
We value your privacy and your rights as a data subject and have therefore appointed Prighter Group with its local partners as our privacy representative and your point of contact.
Prighter gives you an easy way to exercise your privacy-related rights (e.g. requests to access or erase personal data). If you want to contact us via our representative Prighter or make use of your data subject rights, please visit the following website. https://prighter.com/q/11999524960
From time to time, we may change this Policy. We will provide you notice of such changes through the Website interface.
You may contact us through our Website by completing our online form or via email at [email protected], We will do our best to resolve your issue promptly.
Last update: April, 2022
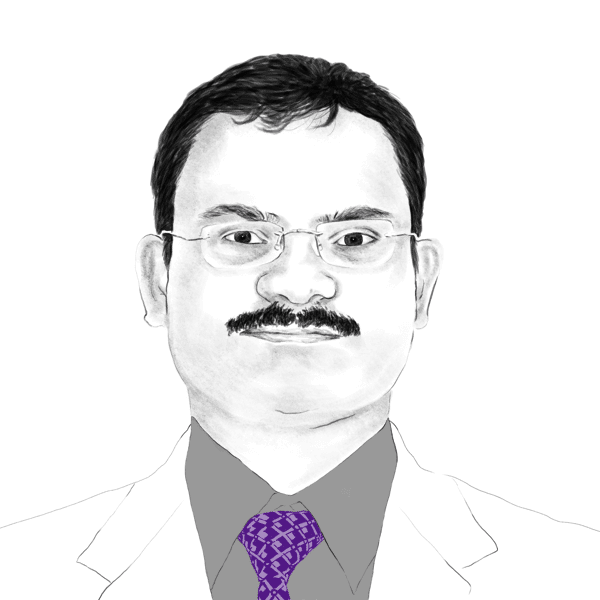
Dr. Raut is Vice President, Global Business Development at Sun Pharmaceuticals Ltd., the largest pharma company in India where he oversees global business development activities for novel specialty branded products. The activities include in-licensing, out-licensing, Mergers and Acquisition, Joint Ventures, research collaborations with academia and pharma/biotech companies, equity investments as well as investments in venture capitals.
Dr. Raut joined Sun Pharma in 2010 and was responsible for clinical research and development for multiple therapy areas including ophthalmology, dermatology and respiratory.
While working in clinical research, he was also involved in multiple business development activities for branded business and is now responsible for global business development of branded products.
He was involved in the global in-licensing for several innovative products such as Ilumya/Ilumetri, Cequa, Yonsa, Odomzo and multiple other pipeline products.
Before joining Sun, Dr. Raut was working at Alcon Laboratories in the US. Dr. Raut holds MD degree from India and PhD degree from USA.

Mrs. Benton has over 30 years of experience in the biomedical industry, as an executive and entrepreneur. For the past four years, she has served as the Head of New Products and Business Development at Shire, where she played an instrumental role in the expansion of the Ophthalmology Franchise pipeline. Before joining Shire, Mrs. Benton worked in Global Business Development at Bausch and Lomb Pharmaceuticals prior to the acquisition of the company by Valeant. Her deal sheet includes over 20 transactions to date. She also co-founded and served as the Chief Commercial Officer for Sirion Therapeutics, a biopharmaceutical company that developed and launched Durezol® and Zirgan® in the US market before being acquired by Alcon and Bausch and Lomb respectively. She has served as a strategic consultant for more than a dozen ophthalmic start-up companies in the areas of clinical development, strategic marketing, and commercial and business operations. Before joining the ophthalmic community, Mrs. Benton worked as Head of Consumer and Professional Sales for J&J Lifescan and in Marketing & Sales for Sanofi Pasteur. Mrs. Benton holds an MBA from the Executive Program at the University of South Florida and a B.S. in Biology from Muhlenberg College.

Mr. Bucher is Co-Founder and Partner at Forty51 ventures, a Biotech VC fund in Europe focused on venture formation. Previously, he was Partner and Managing Director Investments at Roivant and Head of Europe. Before that he worked for Roche in numerous Business and Corporate Development functions including as Deputy Head of Global M&A. He was involved in 100+ transactions and negotiated and closed over 50 international deals with an accumulated total deal value of more than 10 billion USD. Prior to Roche, Sascha was a banker at UBS. He holds an MBA in Finance and Economics from the University of Basel and is both a Harvard Business School GMP alumnus and a Certified European Financial Analyst and Asset Manager (CEFA).
We at Tarsier Pharma Ltd. (“Tarsier Pharma”, “we”, “us” or “our”) respect your privacy. This Privacy Policy (“Policy”) explains the accepted privacy practices for our online services at our website (“https://tarsierpharma.com/”). It also describes the rights and options available to you with respect to your personal information.
The Website provides content and information about Tarsier Pharma and its research and development of a novel platform for treatment of ocular inflammatory diseases.
You do not have a legal duty to provide the information and providing it is subject to your unconditional consent. Proving the service of online inquiries on our Website will not be available to those choosing not to provide the information requested.
We collect two types of information: information you actively provide us when contacting us or statistical information about use of the Website.
Inquiries. If you wish to send us a query of any kind via our online contact form, you will be required to provide us with your name and email address (“Inquiry Information”). In order to respond to certain queries, we may ask you to provide additional information (such as your telephone number).
Statistical information. When you visit our Website, we may record and collect certain information in relation to your visit and use of the Website, including: IP address (and location), time and date of access, type of browser used, links clicked and the web pages you accessed (“Analytical Information”).
Cookies. We collect Analytical Information through “Cookies”, which are text files, comprised of small amount of data, that are saved on your computer or other device (e.g. smartphone, tablet, etc.) when you use internet and visit various websites. We use cookies for several purposes:
Necessary. Cookies that are strictly necessary for the functioning of the Website. The Website cannot operate properly without these cookies. You can set your browser to block or alert you about these cookies, but some parts of the Website may not function properly.
Statistics. Cookies that help us understand how you and other users interact with our Website by collecting data that does not directly identify you.
The overall personal data outlined above will be referred to as the “Information”.
We use your Information in order to contact you about Inquiries you made and/or to operate the Website.
We may use your Information outlined above for the following purposes:
Operating the Website and providing its features and functionalities;
Contact you about inquiries you made;
Send you email communications concerning our products, programs, developments and other related information. We will send you email communication subject to your explicit consent;
You may ‘opt-out’ of using your data for promotional communications at any time by following the “unsubscribe” link located at the bottom of each message. By doing so, Tarsier Pharma will only delete the Information which is required to contact you for promotional communications, while the rest of the Information you submitted to us which is necessary in order to provide you with the Website’s services will continue to be processed.
Improve and enhance the services and content offered on the Website.
We share your Information mainly with our contractors and service providers, strictly for the purpose of helping us with the internal operation of the Website.
We will not share your Information with third parties, except in the events listed below or when you provide your explicit and informed consent:
We will share your Information with our service providers (such as our hosting providers) who assist us with the internal operations of our Website and our services.
These companies are authorized to use your Information only as necessary to provide these services to us and not for their own promotional purposes. We do not sell your Information to third parties
If you have breached any agreement you have with us (including this Policy), abused your rights to use the Websites, or violated any applicable law, your Information may be shared with the competent authorities, law enforcement and third parties (such as legal counsels and advisors), for the purpose of handling of the violation or breach;
If we are required to disclose your Information by a judicial, governmental or regulatory authority;
We will share your Information with members of our family group of companies, who help us process the data for the purpose set out above;
If the operation of the Websites is organized within a different framework, or through another legal structure or entity (e.g. due to a merger or acquisition), we will share your Information to enable the structural change.
We will store your data for as long as we deem necessary for the purposes detailed in this Policy
We retain personal data for as long as necessary to fulfil the purposes we collected it for (as detailed above), including for the purposes of satisfying any legal, accounting, or reporting requirements. We, at Tarsier Pharma, always consider the type of information collected, the amount of information, how sensitive it might be. Accordingly, we determine the appropriate retention period.
We may also anonymize your Information so that it can no longer be associated with you, in which case we will use such Information without further notice to you.
We implement measures to reduce the risks of damage and unauthorized access or use of information, including when data is transferred outside the E.U.
Security Measures. We implement measures to reduce the risks of damage, loss of Information and unauthorized access or use of Information. We also request our affiliates to implement such measures in order to secure the Information we provide them. However, although efforts are made to secure your Information, we cannot guarantee its absolute protection.
Cross-Border Data Transfers. We are based in Israel. Information we collect from you will be processed in Israel, which is recognized by the European Commission as having adequate protection for personal data. Certain information may be stored externally via cloud services. We will only transfer your personal information to a country or company which has been deemed to have an adequate level of data protection by the EU commission, or under other adequate safeguards determined under the applicable law.
Controller. Tarsier Pharma Ltd. is the data controller for the purposes of the personal data we collect via the Website and for the performance of the services offered through the Website.
The legal basis for processing and collecting Inquiry Information is its necessity for the provision of feedback, comment or service in response to your inquiry;
The legal basis for processing and collecting Analytical Information is our legitimate interests in operating our website, ongoing management of our business and business development;
The legal basis for collecting and processing your information for Promotional Communications is your explicit consent;
Right to Access. You have a right to access your personal data that we process and receive a copy of it.
Right to Rectification. You have the right to ask us to rectify inaccurate personal data concerning you and to have incomplete personal data completed.
Right to Data Portability. You have a right to receive the personal data that you provided to us, in a structured, commonly used and machine-readable format. You have the right to transmit this data to a third party. Where technically feasible, you have the right that your personal data be transmitted directly from us to a third party you designated.
Right to Withdraw Consent. You have the right to withdraw your consent for processing your personal data at any time. If you do that, we will not collect any further personal data, but we will further process the data we already collected for reasons described in this Policy. Withdrawing your consent will not affect the lawfulness of data processing we carried out based on your consent before such withdrawal.
Right to Object. If you previously agreed that your personal data may be used for other purposes other than registering and/or placing an order, you may have a right to object to the use of your personal data for such additional purposes.
Right to Restrict. You have the right to restrict processing of your personal data (except for storing it) if you contest the accuracy of your personal data, for a period enabling us to verify its accuracy; if you believe that the processing is unlawful and you opposes the erasure of the personal data and requests instead to restrict its use; if we no longer need the personal data for the purposes outlined in this Policy, but they are required by you to establish, exercise or defence relating to legal claims, or if you object to processing, pending the verification whether our legitimate grounds for processing override yours.
Right to be Forgotten. Under certain circumstances, such as when you withdraw your consent, you have the right to ask us to erase your personal data. However, we may still process your personal data if it is necessary to comply with a legal obligation we are subject to under laws in EU Member States.
If you believe your right have been infringed, you can lodge a complaint with a supervisory authority operating under the GDPR. For a list of supervisory authorities in the EU, click here.
Representation for data subjects in the EU
We value your privacy and your rights as a data subject and have therefore appointed Prighter Group with its local partners as our privacy representative and your point of contact.
Prighter gives you an easy way to exercise your privacy-related rights (e.g. requests to access or erase personal data). If you want to contact us via our representative Prighter or make use of your data subject rights, please visit the following website. https://prighter.com/q/11999524960
From time to time, we may change this Policy. We will provide you notice of such changes through the Website interface.
You may contact us through our Website by completing our online form or via email at [email protected], We will do our best to resolve your issue promptly.
Last update: April, 2022
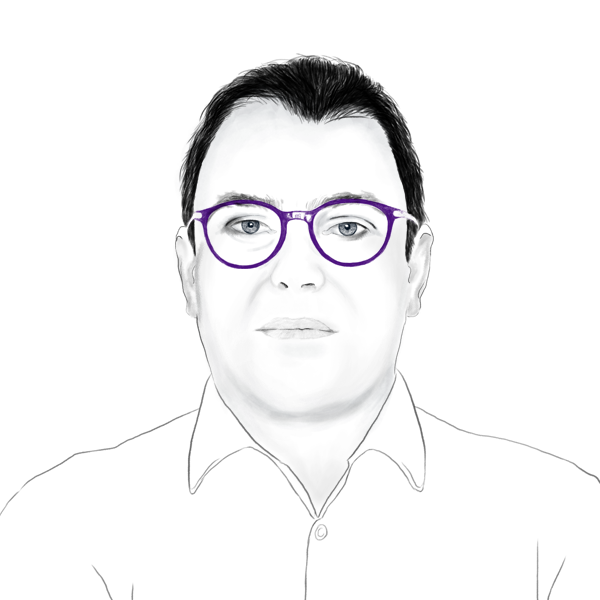
Dr. Defert has over 15 years of experience in the industry. In his most recent position, Dr. Defert co-founded Amakem NV and Ophthakem NV, where he also served for 8 years as Head of R&D and member of the executive management team. In these positions, he led IND-enabling studies contract research management, medicinal chemistry and projects management of a novel locally acting Rho kinase inhibitor for the treatment of glaucoma – with Clinical Phases 2 completed or ongoing in different countries. Olivier holds a Ph.D. in medicinal chemistry, DeVGen NV and the Laboratoire de Chimie des Biomolécules (Lille). He is the author of 19 patent applications and 10 peer-reviewed publications.

Dr. Haim-Langford is the founder and CEO of Tarsier Pharma. She has over 20 years of experience in the biomedical industry, as an investor, entrepreneur and executive. Prior to founding Tarsier Pharma, she served as VP business development at Xenia venture capital, where she managed the life sciences practice, led investments in the medical technology and biotechnology space, and served on the boards of pharmaceutical, medical device and biotechnology companies in various stages of product development (from early pre-clinical through clinical development, up to sales & marketing, M&A deals). Few examples include Meditate (Acquired by Olympus for up to $285 million), Orthospace (Acquired by Stryker for up to $220 million), Omnix Medical, and others. At Xenia, she also co-founded and served as the Chairperson of the board at Eximore, an ophthalmic drug delivery company.
Before joining Xenia, Dr. Haim-Langford was VP Business Development at Medingo (Acquired by Roche for $200M million). She co-founded the Israeli Biomimicry Organization, a non-profit organization aimed to increase awareness to the biomimicry discipline and drive inspiration by nature as a source for biomedical innovations. Dr. Haim Langford holds a Ph.D. in Biophysics from the Technion, the Israeli Institute of Technology.

Ms. Milman brings hands-on experience from drug development projects, among them Orphan Technologies, a company focused on development of novel drugs for rare, life-threatening diseases that was acquired by Retrophin (NASDAQ: RTRX) in a deal worth $517M. On top of that, Ms. Milman brings diverse experience from a venture capital firm, where she managed due diligence processes for hundreds of early-stage projects, and establishment of early-stage medical device and pharmaceutical start-up companies.
Ms. Milman has been instrumental in Tarsier Pharma’s speedy and successful evolvement, as she heavily contributed to bringing Tarsier from pre-clinical PoC to a phase-3 late-stage pharmaceutical company, in only 5 years. As COO of the company since its inception, Ms. Milman managed numerous aspects of corporate activity, from R&D, pre-clinical, CMC, through regulation and patents, while supporting clinical, legal, business development, marketing and fund raising activities.
Ms. Milman holds an M.Sc. in Biomedical Sciences and Human Genetics from the Hebrew University of Jerusalem, Israel.

Dr. Neumann is an ophthalmologist, specializing in ocular immunology & clinical uveitis. He specialized in Inflammatory Eye Diseases (IED) at Mass Eye and Ear Infirmary, Harvard University and was formerly the Head of IED at Sheba Hospital. Together with his clinical expertise, he brings vast experience from the pharmaceutical industry as former Head of global medical affairs and ophthalmology clinical development at Teva Pharmaceutical Ltd. He was involved in the early stage of a small company (Pharmos) as their head of ophthalmic development, and was later leading contract research within an HMO (Maccabi healthcare organization). Since 1995, he is the Founder and co-chairman of the international Symposium of Ocular Pharmacology and Therapeutics, ISOPT Clinical.

Dr. Quan Dong Nguyen currently is a Professor of Ophthalmology at the Byers Eye Institute, Stanford University School of Medicine.
Dr. Nguyen received his Bachelor and Master of Science degrees simultaneously in Molecular Biophysics and Biochemistry from Yale University, and earned his medical degree at the University of Pennsylvania School of Medicine. He completed an internship in Internal Medicine at the Massachusetts General Hospital and a residency in Ophthalmology at the Massachusetts Eye and Ear Infirmary, Harvard Medical School. Dr. Nguyen also completed fellowships in Immunology and Uveitis at the Massachusetts Eye and Ear Infirmary, Ocular Immunology at the Wilmer Eye Institute of the Johns Hopkins Medical Institutions, and medical and surgical retina at the Schepens Eye Research Institute and the Massachusetts Eye and Ear Infirmary.
In 2001, Dr. Nguyen joined the faculty at the Wilmer Eye Institute, Johns Hopkins University School of Medicine, as Assistant Professor and then Associate Professor of Ophthalmology and Director of Medical Education. In 2013, he was appointed as the McGaw Endowed Chair in Ophthalmology, Professor and Chairman of the Department of Ophthalmology and the Inaugural Director of the Stanley M. Truhlsen Eye Institute, and Assistant Dean for Translational Research at the University of Nebraska Medical Center.
Dr. Nguyen serves as principal investigator on multiple clinical trials sponsored by the National Eye Institute and other organizations for macular edema (from diabetes and uveitis), neovascular age-related macular degeneration (AMD), and ocular inflammatory and uveitic diseases, as well as co-investigator on numerous other clinical trials involving novel therapeutic agents. Dr. Nguyen is known for his innovative work in early proof-of-concept, first-in-human clinical trials to evaluate potential pharmacotherapeutic agents for retinal vascular and uveitic diseases. Dr. Nguyen and his team were among the first clinician scientists in the world to evaluate aflibercept for neovascular AMD and ranibizumab for diabetic macular edema (DME); the initial results of these studies served as the foundation for subsequent trials leading to the approval of these pharmacologic agents by the FDA and other regulatory authorities for the indicated diseases. Dr. Nguyen has chaired the United States multi-center READ-2, READ-3, and iDEAL studies, evaluating the potential role of VEGF antagonists, through different pathways, for diabetic macular edema.
Dr. Nguyen has lead the SAVE, and the multi-centered SAVE-2, and STOP-UVEITIS studies to evaluate the role of new pharmacologic agents, including specific interleukin inhibition, in uveitis and ocular inflammatory diseases.
At the Byers Eye Institute at Stanford, Dr. Nguyen has an active uveitis and ocular inflammatory diseases as well as clinical and surgical retina practice while he continues his research in pharmacotherapy and ocular imaging. In addition, he teaches and trains students, residents, and clinical and research retina and uveitis fellows at Stanford.

Prof. de Smet completed his medical training in McGill and UBC. He worked for 8 years at the NEI in Bethesda MD in the immunology service alongside Robert Nussenblatt, completing his term as Chief of the Clinical Immunology Service. He completed a 1-year fellowship at the Wilmer Eye Institute in Vitreo-retinal surgery in Baltimore.
Marc served for 10 years as head of the department of Ophthalmology at the University of Amsterdam, where he initiated a clinical research unit and forged collaborations with outside departments including the biomedical engineering department of the Technical university in Eindhoven, the Netherlands. He has since moved to a private group practice focused on retina and inflammatory disorders in Lausanne, Switzerland.
His research interests include retinal imaging (during which he pioneered with Richard Rosen, transverse scanning); pharmacology as applied to retinal diseases, leading for example to the clinical development of Jetrea (ocriplasmin); the use of methotrexate in eyes for the treatment of ocular lymphoma; the use of steroids for various ocular indications. For 10 years, he has worked on the development of a robotic surgical platform for eye surgery along with the bio-engineering group at TU/e leading to Preceyes bv, a start-up dedicated to the development of high precision assistive eye surgery. He is the current chief medical officer of the company. The robotic arm was used for the first time in humans August 2016.
He has authored and co-authored more than 175 papers, 25 books and book chapters, given numerous lectures and named lectures. Treasurer of the international uveitis study group, he is member of the Jules Gonin club, the macula society and heads a division of the EViCR.net – a European clinical research network.
Dr. Cunningham serves as Managing director at Clarus ventures. He joined Clarus in 2006 with extensive experience in the biomedical and biopharmaceutical sectors. Prior to joining Clarus, Dr. Cunningham was the Senior Vice President, Medical Strategy at Eyetech Pharmaceuticals, Inc. (NASDAQ:EYET), where he helped build and lead the team that developed and commercialized Macugen, a first-in-class product for the treatment of age-related macular degeneration. Prior to Eyetech, Dr. Cunningham was at Pfizer, Inc (NYSE: PFE), where he was responsible for the clinical development of early phase central nervous system compounds and the in-licensing of early and late-stage therapeutic candidates in ophthalmology.
Dr. Cunningham is an internationally recognized specialist in infectious and inflammatory eye disease with over 350 publications. He is Adjunct Clinical Professor of Ophthalmology at Stanford University School of Medicine, was Clinical Professor and Director of the Uveitis service at NYU from 2002 to 2005, and was Director of both the Uveitis Service and the Kimura Ocular Immunology Laboratory at the University of California at San Francisco (UCSF) from 1995 to 2001. Dr. Cunningham received an MD and MPH in epidemiology and statistics from Johns Hopkins University and a PhD in neuroscience from the University of California at San Diego (UCSD) for work done at The Salk Institute. He completed both a residency in ophthalmology and fellowship training in Corneal Disease and Uveitis at UCSF and The Francis I. Proctor Foundation, a medical retina and uveitis fellowship at Moorfields Eye Hospital in London, and a fellowship in public health ophthalmology at the Wilmer Eye Institute in Baltimore.
Dr. Cunningham founded and is the Chairman of the Ophthalmology Innovation Summit, a symposium held in conjunction with the annual meetings of the American Academy of Ophthalmology, the American Society of Cataract and Refractive Surgery and the American Society of Retinal Surgeons.
Dr. Cunningham represents Clarus on the Board of Directors of Annexon Biosciences, Graybug Vision, Restoration Robotics, and SFJ Pharmaceuticals Group. He is a Board observer for Lumos Pharma and is on the Scientific Advisory Board of Aerie Pharmaceuticals (NASDAQ: AERI).
Previous Directorships include Neomend (acquired by Bard) and SARcode Biosciences (acquired by Shire). Additionally, he was Board Observer for Avillion, Ferrokin (acquired by Shire), Ophthotech (NASDAQ: OPHT), Pearl Therapeutics (acquired by AstraZeneca), and was a member of the Scientific Advisory Board for ESBATech (acquired by Alcon).